PDE4 inhibitor overview
In development for atopic dermatitis in patients aged 2-5.
Details
Topical roflumilast is a selective and highly potent,* nonsteroidal phosphodiesterase 4 (PDE4) inhibitor being investigated for the once-daily treatment of atopic dermatitis in patients aged 2-5.1
- Topical roflumilast is being investigated as a cream formulation
- Topical roflumilast is a highly potent, anti-inflammatory PDE4 inhibitor reformulated from an oral product that was approved by the US Food and Drug Administration in 2011 to treat patients with severe chronic obstructive pulmonary disease2
*In vitro data. Clinical efficacy claims cannot be made.
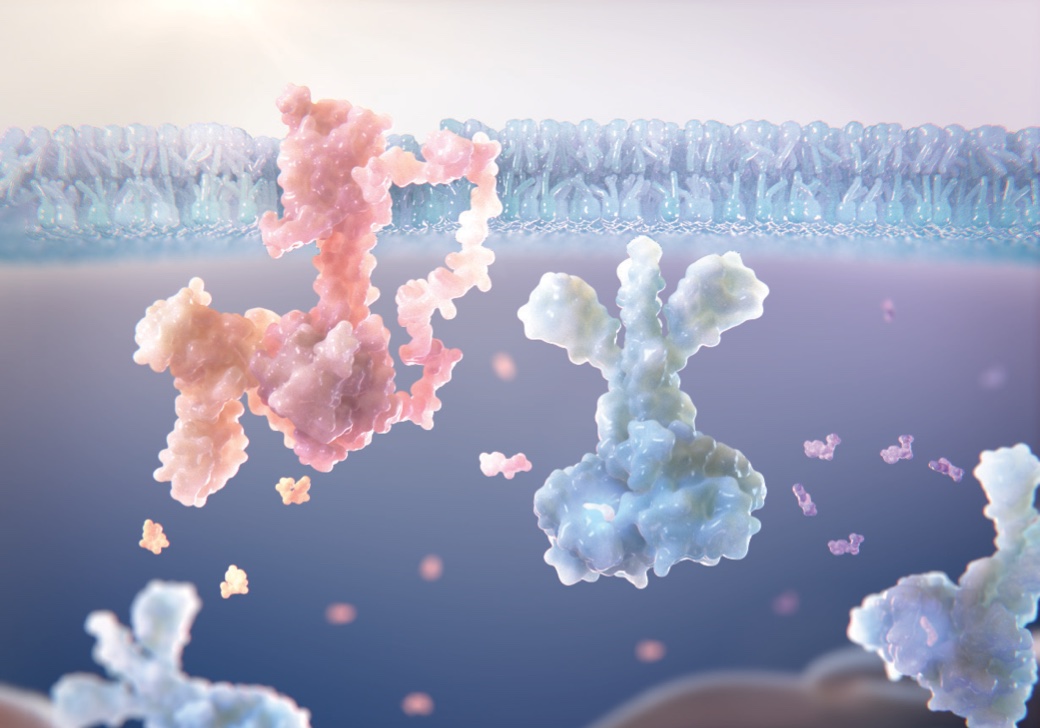
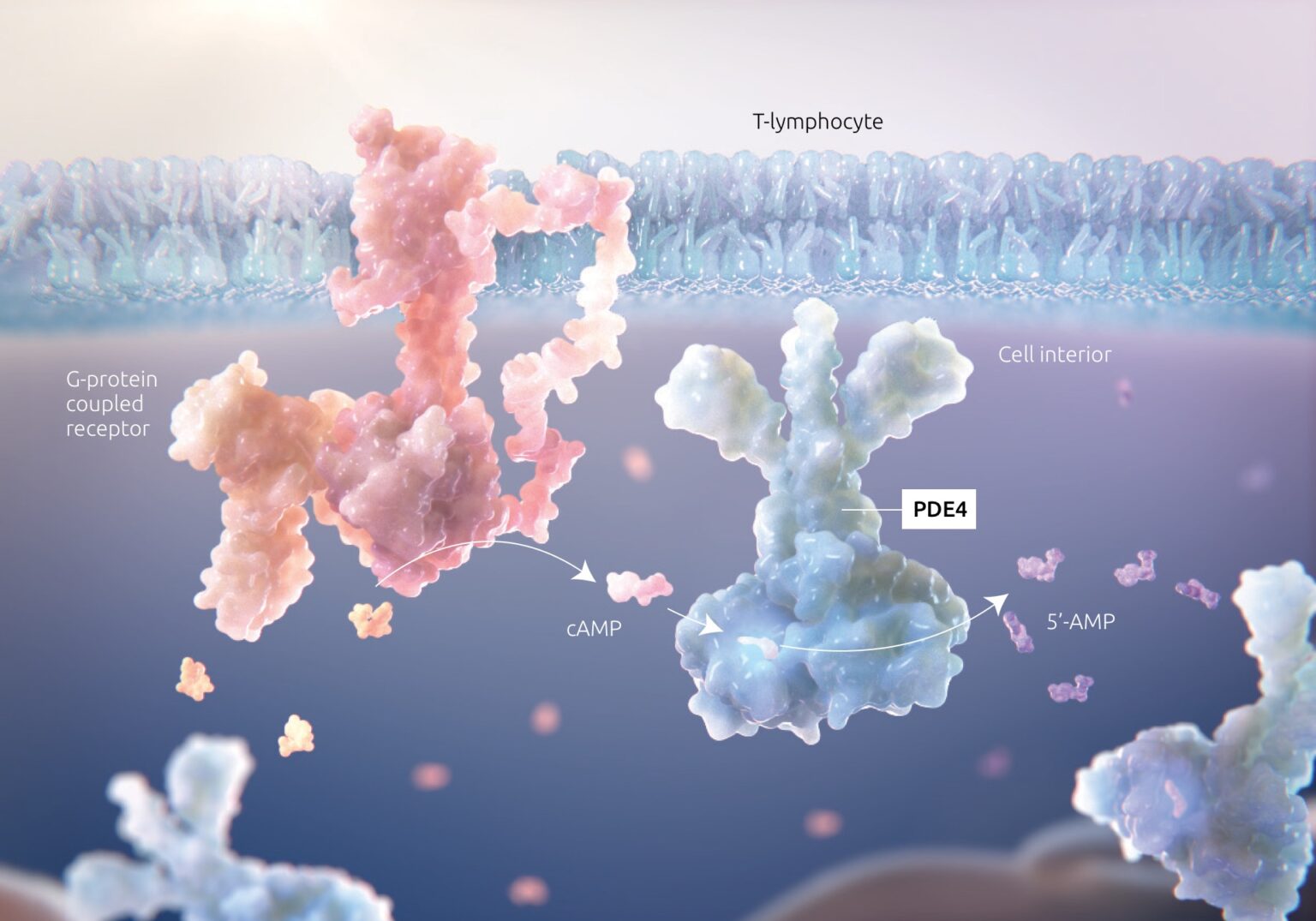
PDE4 plays a key role in cutaneous immunology2
PDE4 is an enzyme that contributes to immune-mediated dermatologic conditions.


Inhibition of PDE4 modulates the immune response in the skin1,2
Inhibition of PDE4 modulates the immune response by downregulating key inflammatory cytokines such as IFN-γ, TNF-α, IL-4, IL-17, and IL-23; restoring the skin barrier balance, and decreasing inflammatory response.

IFN = interferon, IL = interleukin, TNF = tumor necrosis factor.
Therapy
Roflumilast is a PDE4 inhibitor being investigated in a topical cream formulation.
References
1. Dong et al. J Pharmacol Exp Ther. 2016;358(3):413-422. 2. Li et al. Front Pharmacol. 2018;9:1048. 3. Data on File. Arcutis Biotherapeutics, Inc. 4. Berk et al. Presented at: The Society for Investigative Dermatology Annual Meeting; May 18-22, 2022; Portland, OR.