Roflumilast cream (ARQ-151)
Overview
Roflumilast cream (ARQ-151) is being investigated for the treatment of atopic dermatitis.
Roflumilast cream is uniquely formulated as an emollient, water-based (~48% water) cream without fragrances, propylene glycol, isopropyl alcohol, or ethanol. It contains a novel emulsifier designed to maintain epidermal intercellular lipids and is adjusted to a stratum corneum pH at which the skin normally functions. Ongoing clinical trials are investigating roflumilast cream 0.15% and 0.05% in patients with atopic dermatitis.
Atopic dermatitis
Roflumilast cream (ARQ-151) in atopic dermatitis.
Clinical landscape
- Atopic dermatitis is a common, chronic inflammatory skin disease affecting both children and adults1
- Itch is the most burdensome symptom, causing patients to experience substantially reduced quality of life and sleep disturbances2
- The skin barrier is typically compromised in atopic dermatitis3
- Topical corticosteroids and emollients are the standard of care3
- Steroids are not suitable for long-term use, and children are at risk of greater systemic absorption3
Proposed MOA
- Elevated PDE4 levels are found in the keratinocytes of individuals with atopic dermatitis3
- Inhibition of PDE4 decreases inflammatory response and pruritus3,4
- Roflumilast cream (ARQ-151) is a selective, highly potent* PDE4 inhibitor being investigated as a treatment for atopic dermatitis5
*In vitro data. Clinical efficacy claims cannot be made.
MOA = mechanism of action
Clinical programs
Phase 3
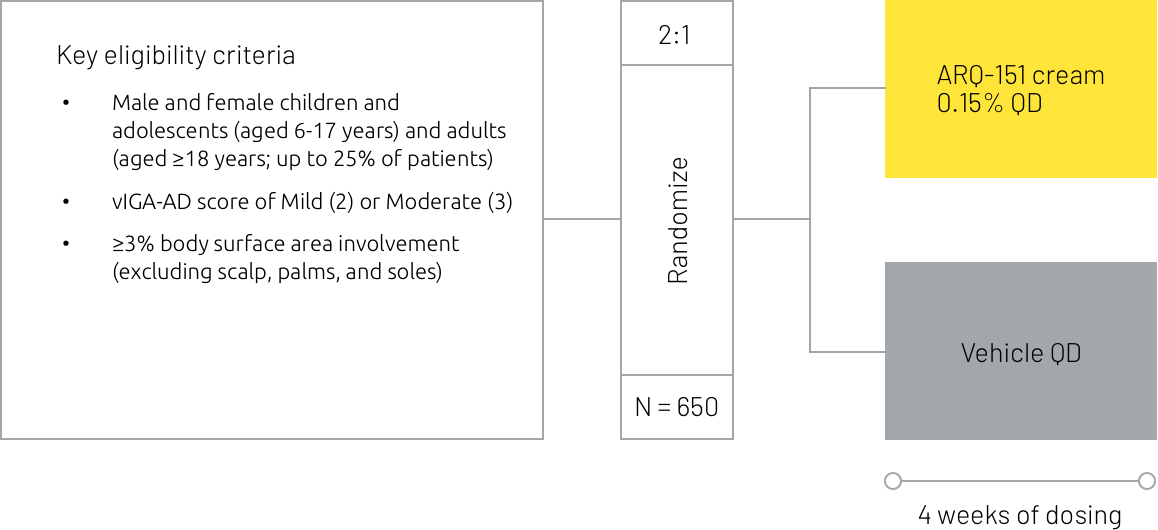
Two phase 3 trials (INTEGUMENT-1 and -2) have been completed for roflumilast cream in atopic dermatitis.
ClinicalTrials.gov identifiers: NCT04773587 (INTEGUMENT-1) and NCT04773600 (INTEGUMENT-2)
Study design summary6
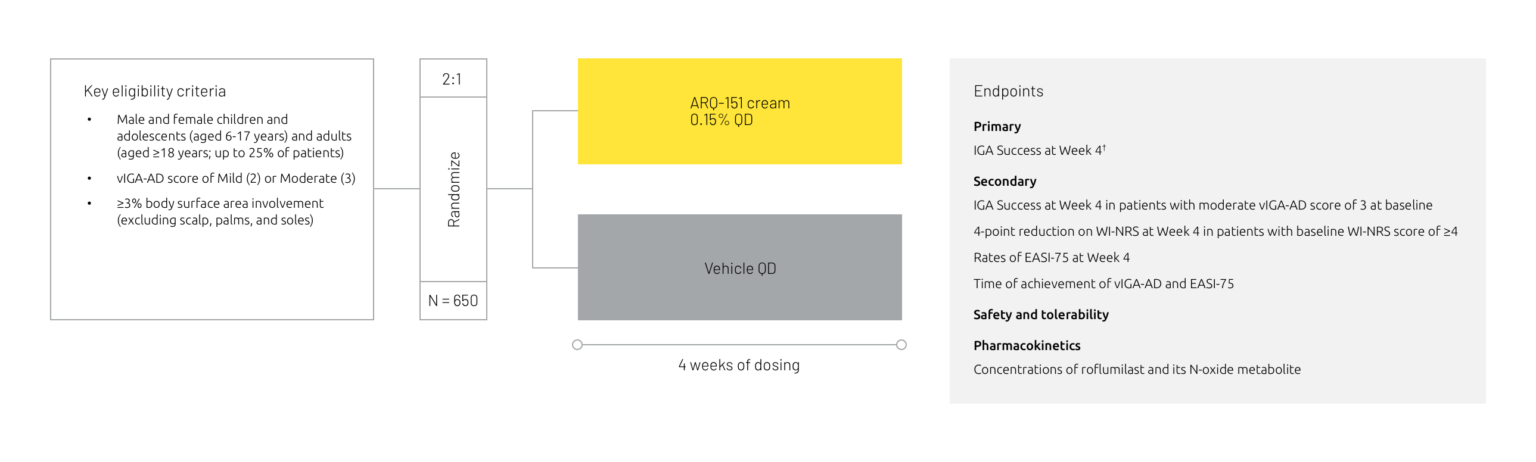
†IGA Success was defined as a score of 0 or 1 (Clear or Almost Clear) with at least a 2-grade improvement from baseline.
EASI-75 = Eczema Area and Severity Index 75% improvement, IGA = Investigator’s Global Assessment, QD = once daily, vIGA-AD = Validated Investigator’s Global Assessment for Atopic Dermatitis, WI-NRS = Worst Itch Numeric Rating Scale.
Phase 3
A phase 3 trial of roflumilast cream 0.05% in pediatric patients aged 2-5 years is ongoing (INTEGUMENT-PED).
ClinicalTrials.gov identifier: NCT04845620
Phase 3
A phase 3 long-term study is ongoing (INTEGUMENT-OLE).
ClinicalTrials.gov identifier: NCT04804605
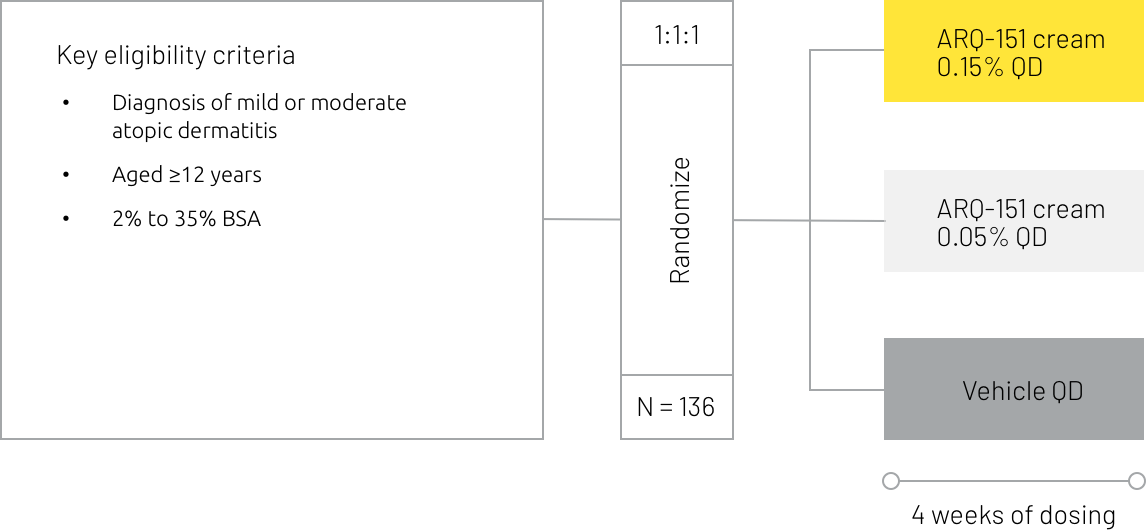
Phase 2
A phase 2 trial assessing the safety and efficacy of ARQ-151 cream in patients with atopic dermatitis has been completed.
ClinicalTrials.gov identifier: NCT03916081
Study design summary7
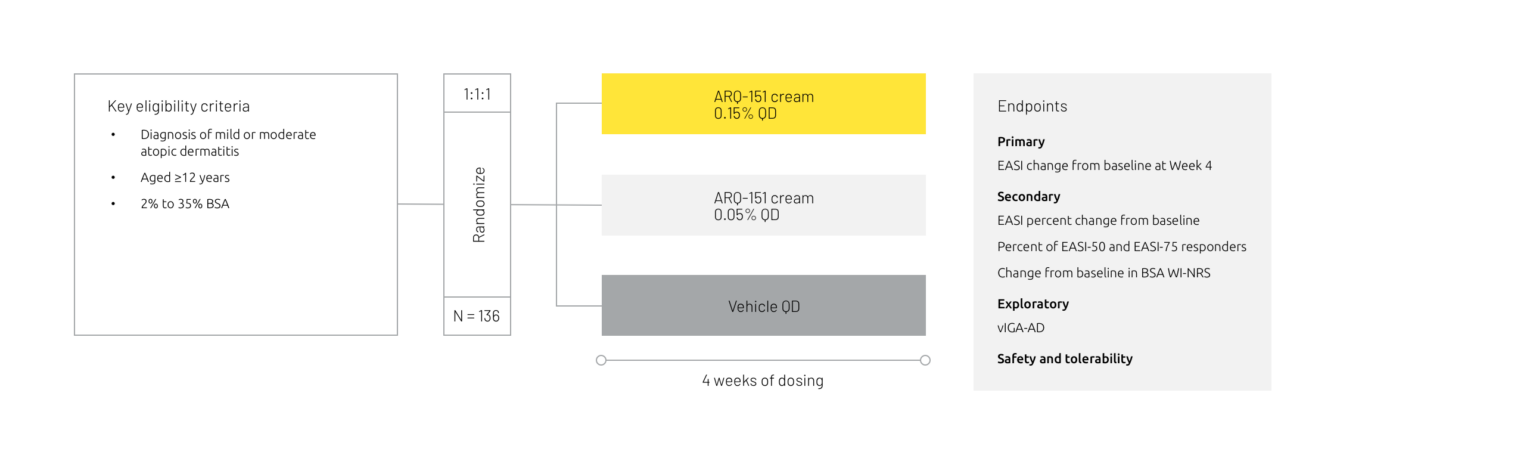
BSA = body surface area, EASI = Eczema Area and Severity Index, EASI-50 = Eczema Area and Severity Index 50% improvement, EASI-75 = Eczema Area and Severity Index 75% improvement, IGA = Investigator’s Global Assessment, QD = once daily, vIGA-AD = Validated Investigator’s Global Assessment for Atopic Dermatitis, WI-NRS = Worst Itch Numeric Rating Scale.
References
1. Bieber. Ann Dermatol. 2010;22:125-137. 2. Silverberg et al. Ann Allergy Asthma Immunol. 2018;121:340-347. 3. Nygaard et al. Dermatology. 2017;233:333-343. 4. Bäumer et al. Inflamm Allergy Drug Targets. 2007;6:17-26. 5. Dong et al. J Pharmacol Exp Ther. 2016;358:413-422. 6. Data on File. Arcutis Biotherapeutics, Inc. 7. Gooderham et al. Presented at: 29th European Academy of Dermatology and Venereology Congress; October 29-31, 2020; Virtual.